Increased intestinal permeability has been studied in the context of inflammatory bowel disease, graft-versus-host disease; in vivo studies have shown that this barrier loss promotes disease progression. Although more subtle permeability increases have been associated with irritable bowel syndrome (IBS), depression, stress-related disorders, and autism spectrum disorder, no studies have examined whether barrier loss can be a primary cause of or otherwise contribute to pathogenesis of these disease.
This work, the first to implicate physiological-range increases in intestinal epithelial tight junction permeability, made use of transgenic mice. These mice express a constitutively-active myosin light chain kinase within the intestinal epithelium. This triggers a well-characterized physiological and pathophysiological mechanism of tight junction barrier regulation.
Previous work has shown that there is mild intestinal mucosal immune activation in these constitutively-active myosin light chain kinase transgenic mice, and this immune activation both limits early pathogen invasion and accelerates progression of immune-mediated colitis. However, in the absence of exogenous challenges, the transgenic mice are healthy and do not display obvious defects in growth and development, reproduction, or aging.
This study made use of high-resolution approaches to determine the potential impact of physiological-range intestinal permeability increases on phenotypes linked to disorders that have been clinically-associated with modest barrier loss but do not have overt inflammatory or other anatomic defects.
The open-box study, in which mice are placed in a box and the fraction of time spent near the walls or in the middle of the box, i.e., open space, is assessed, has been used as a measure of anxiety-like behavior. Both time spent and distance traveled within the open space were reduced by ~50% in transgenic mice relative to littermate controls. These are the first data to demonstrate that direct, physiological-range intestinal barrier loss can trigger behavioral changes.
In further studies, a catheter was place within the intestine of transgenic mice and littermate controls. Neuronal signaling was measured during catheter inflation, i.e., intestinal distension. Neuronal responses in control and transgenic mice differed markedly, showing, for the first time, that intestinal epithelial tight junction permeability can modify peripheral neurological responses to physical perturbation of the intestine.
Finally, the impact of physiological-range permeability increases on signaling within the brain. Transgenic mice displayed increased neuronal activation in the thalamus, hypothalamus, and hippocampus, but not other sites. These data suggest that intestinal epithelial tight junction barrier loss can trigger a neurological, systemic stress response that recapitulates some of the changes documented in seen in patients with irritable bowel syndrome, depression, stress-related disorders, and autism spectrum disorder.
Separate analyses also showed that, relative to littermate controls, the microbiome was perturbed by transgenic constitutively-active myosin light chain kinase expression in the intestinal epithelium. Specific changes included increased Clostridium and decreased Bacteroidetes, Enterococcus spp, and Prevotella. Similar shifts occur in patients and have been referred to as dysbiosis.
Overall, these data show that isolated, physiological-range increases in intestinal permeability can trigger phenotypes associated with irritable bowel syndrome, depression, stress-related disorders, and autism spectrum disorder. It is not known, for example, if dysbiosis causes activation of stress pathways in the brain to cause anxiety-like behavior and altered sensitivity to intestinal distension or, alternatively, if the microbial changes are secondary to stress pathway activation. Thus, future work will be needed to understand the relationships between these separate events. Nevertheless, this study shows that direct perturbation of intestinal epithelial tight junction permeability is sufficient to initiate a progress that leads to diverse, subtle phenotypes associated with range of human disorders.
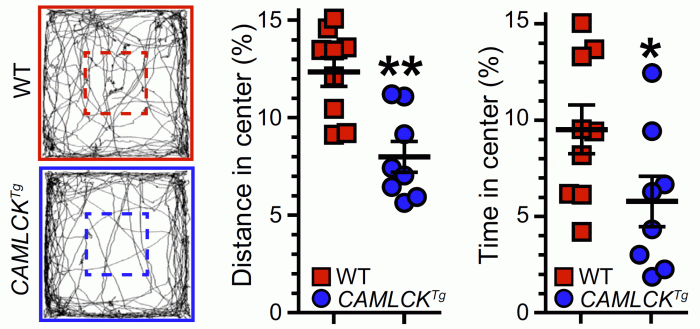
Videotracking paths of representative control (red box) and transgnic (blue box) mice in the open field test. Percent distance traveled in the center (marked by dashed lines) and percent of time in the center are shown for control (red squares) and .transgenic (blue circles) littermates.
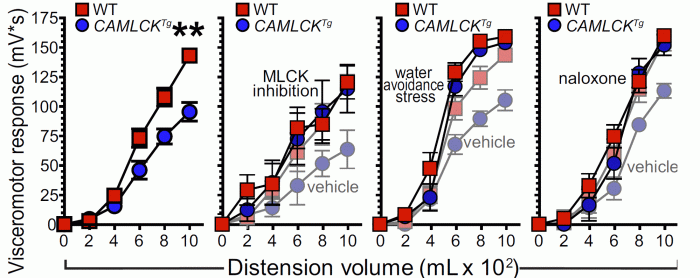
Stepwise colorectal distension-induced visceromotor responses in control (red squares) and transgenic (blue circles) littermates. Transgenic mice displayed visceral analgeisa that was relieved by myosin light chain linase inhibition, stress (water avoidance), or naloxone (an opioid anatagonist). Vehicle controls CAMLCKTg shown with pale symbols in the last three graphs.
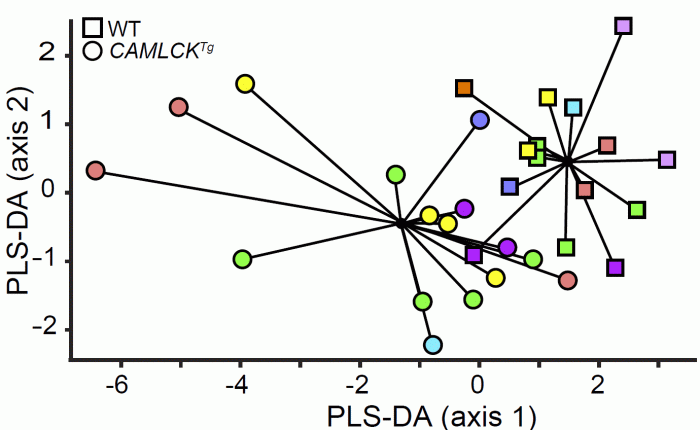
Partial least squares discriminant analysis (PLS-DA) plot of
the relative abundances of 18 microbial taxa in gut contents of control (squares) and transgenic (circles) littermates born to 8 different non-transgenic female mice (each color represents one litter). Microbiota of transgnic mice segregate as a group and are separate from their control (non-transgenic) littermates.
Jerrold R. Turner, MD, PhD
Professor of Pathology and Medicine

Brigham and
Women's
Hospital

Harvard
Medical
School
HNRB 752
77 Avenue Louis Pasteur
Boston, MA 02115
© Copyright JR Turner 2020.